Abstract
Background: Central nervous system lymphoma (CNSL) is a rare but lethal type of non-Hodgkin lymphoma (NHL), including primary CNSL (PCNSL) and secondary CNSL (SCNSL) (Zorofchian et al. Frontiers in Oncology. 2018). High-dose (HD) methotrexate (MTX, M)-based regimens followed by autologous stem cell transplantation (ASCT) or whole-brain radiotherapy (WBRT) (Young et al. Clin Lymphoma MyelomaLeuk. 2020) constitute the main therapeutic strategies. However, the outcome remains poor; nearly 50% of patients had relapsed disease, and 10%-15% were primary refractory (Dasgupta et al. Leuk Lymphoma. 2018; Sacks et al. Int J Stroke. 2018). Recently, several studies have shown that the B-cell receptor (BCR) signaling pathway is more susceptible to mutations in PCNSL (Zhang et al. EHA. 2022). Bruton tyrosine kinase (BTK) is a fundamental component in the BCR pathway. Orelabrutinib is a novel and potent irreversible BTK inhibitor with high cerebrospinal fluid (CSF) concentration and has shown efficacy in the treatment of CNSL (Wu et al. Invest New Drugs. 2022; Yang et al. Front Oncol. 2022; Zhang et al. HemaSphere. 2022). Thiotepa inhibits tumor growth by disrupting DNA bonds showing promising activity in CNSL. Thus, we conducted a retrospective study to evaluate the efficacy and safety of the thiotepa, orelabrutinib, and methotrexate combined with or without the rituximab (TOM±R) regimens in the treatment of patients with CNSL.
Methods: Between March 2021 and March 2022, 13 patients with CNSL who received TOM±R regimens (thiotepa, 30-40 mg/m2, d4; orelabrutinib, 150 mg, daily; methotrexate, 3.5g/m2, d1; rituximab, 375mg/m2, d0; 21 days/cycle) as induction therapy was included in this retrospective study. Dose modification was administered in old patients or patients with renal impairment. After 4 to 6 cycles of the combination treatment, patients could receive consolidation therapy as ASCT or WBRT with or without afterward orelabrutinib maintenance. The primary endpoint was the best overall response rate (ORR) during the induction therapy according to the International PCNSL Collaborative Group (IPCG) standard, and the secondary endpoints included complete response (CR), partial response (PR), progression-free survival (PFS), and adverse events (AEs).
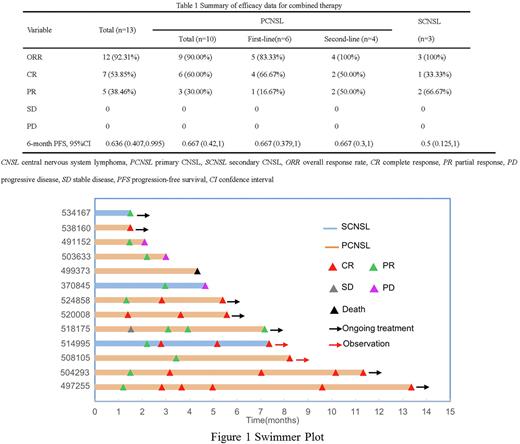
Results: Thirteen patients (6 males; median age 58 years, range, 52-64) with histologically confirmed diffuse large B-cell lymphoma were included in this retrospective study, of whom 6 patients with PCNSL were treatment-naïve, 4 patients with PCNSL were first relapsed, and the rest 3 patients received their first treatment for SCNSL. Most patients had a history of Hepatitis virus infection (n=7), International Extranodal Lymphoma Study Group (IELSG) scores ≥ 2 (n=12), high-risk Memorial Sloan Kettering Cancer Center (MSKCC) score (n=11) and deep intracranial lesions (n=12).The median treatment cycle was 3 cycles (range, 2-9), and 7 patients received TOM+R. The ORR/CR and 6-month PFS rate was 92.3%/53.9% and 63.6% in all patients, 83.3%/66.7% and 66.7% in patients with PCNSL receiving first-line therapy, 100.0%/50.0% and 66.7% in patients with PCNSL receiving second-line therapy, and 100.0%/33.3% and 50% in patients with SCNSL receiving first-line therapy (Table 1). Among the 13 patients, 5 patients received orelabrutinib maintenance treatment, of whom 1 patient underwent WBRT and 1 patient underwent ASCT before orelabrutinib maintenance treatment. The ORR/CR and 6-month PFS rate of the 5 patients receiving orelabrutinib as maintenance treatment were 100%/80% and 100%, respectively (Figure 1). The most commonly reported AEs of all patients included leukopenia (53.9%) and pneumonia (15.4%). Grade 3-4 AEs included white blood cell count decreased (23.1%), thrombocytopenia (7.7%), fever (7.7%), and pneumonia (7.7%). No patients experienced severe AEs, and 1 patient died due to progressive disease.
Conclusion: TOM±R regimens were effective and well-tolerated for patients with CNSL in this retrospective study. This combo regimens may provide a potential treatment strategy for patients with CNSL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal